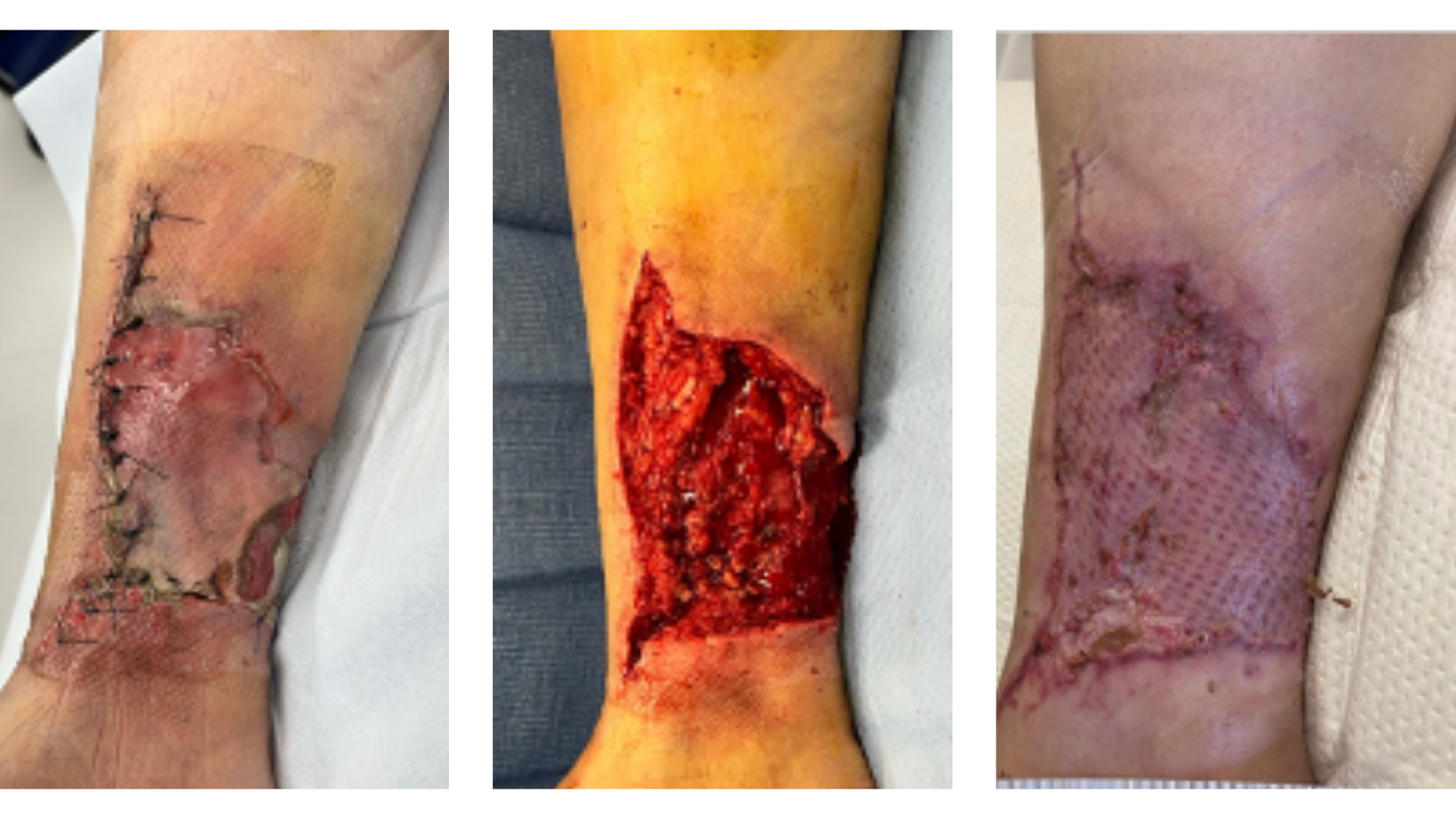
MiroDry™ Wound Matrix
MiroDry™ Wound Matrix, a thinner version of Miro3D, is a hepatic-derived, porous and compressible wound matrix. It is designed to conform to any wound bed, tunneling, and irregular wound beds. Supplied sterile.
Reprise Biomedical
Reprise Biomedical is focused on the development and commercialization of biological matrices that offer advanced solutions for healing. Their products are derived from porcine liver utilizing a patented perfusion decellularization technology.Benefits
• 1cm thick porcine collagen sheet scaffold
• Compressible and conformable to irregular wound bed topography
• Naturally retained porosity provides a protective environment for wound management
Indications
MiroDry™ Wound Matrix is indicated for the management of wounds, including: partial- and full-thickness wounds; pressure ulcers; venous ulcers; chronic vascular ulcers; diabetic ulcers; tunneled, undermined wounds; trauma wounds (abrasion, lacerations, partial-thickness burns, skin tears); drainage wounds; and surgical wounds (donor sites/grafts, post–Mohs surgery, post-laser surgery, podiatric, wound dehiscence).
Contraindications
This device is derived from a porcine source and should not be used in patients with known sensitivity to porcine material. This device is not indicated for use in third-degree burns.
Warnings and Precautions
MiroDry™ Wound Matrix is supplied sterile for single use only. Reuse of a single-use device creates a potential risk of patient or user infections and may compromise the device functionality, which may lead to illness or serious injury.
Do not resterilize, as the safety and performance has not been evaluated for this scenario. This is a single-use device.
Reuse of this device creates a potential risk of patient infections.
Do not use a device past the expiration date as the safety and performance has not been evaluated for this scenario.
Do not use if the package or seal is opened, damaged, or compromised. A damaged package could result in a breach of sterility or device damage, which may lead to illness or serious injury.
After use, handle and dispose of all unused product and packaging in accordance with accepted medical practice and applicable local, state, and federal laws and regulations. This is a single-use device. Reuse of this device creates a potential risk of patient infections.
Adverse Effects/Reactions
The following complications are possible with the use of wound dressings. If any of these conditions occur, the device should be removed: allergic reaction; excessive redness, pain, swelling, or blistering; fever; infection; chronic inflammation; non-healing wound.
Storage Requirements
MiroDry™ Wound Matrix, is a sterile medical device that should be stored in a clean, dry location at room temperature, in its original package. Avoid prolonged exposure to elevated temperatures as it may compromise device functionality.
The product expiration date is indicated as year (4 digits), month (2 digits), and day (2 digits).
How Supplied/Sizing
HCPCS Code
Product features
Other features
Recommended Use
Acute Wounds
Chronic Wounds
Deep Wounds
Diabetic Foot
Pressure Ulcers
Venous Ulcers
Mode of Use/Application
Prepare the wound using standard methods ensuring that the wound is free of debris and devitalized
tissue. An initial debridement of the wound may be necessary to ensure the wound edges contain viable tissue.
MMiroDry™ Wound Matrix should be placed in maximum possible contact with healthy, well-vascularized tissue as a scaffold which provides a protective environment for the wound.
If desired, secure MiroDry™ Wound Matrix with the physician’s preferred fixation method.
Use an appropriate non-adherent primary wound dressing over MiroDry™ Wound Matrix to prevent it
from adhering to the dressing and to protect the integrity of the applied product and not disrupt the wound site.
Apply an appropriate secondary dressing that will manage the wound exudate, keep Miro3D® Wound
Matrix moist, and keep all layers securely in place.
Discard any unused portion of the MiroDry™ Wound Matrix product and the package in accordance with accepted medical practice and applicable local, state, and federal laws and regulations.
As healing occurs, sections of MiroDry™ Wound Matrix may gradually peel. Carefully remove any
remaining loose product around the edge as needed. Do not remove any remaining MiroDry™ Wound
Matrix that has integrated.
If the wound is free of infection and necrosis but not fully epithelialized, follow standard clinical protocol for additional application or therapy.
Removal & Change Frequency
As healing occurs, sections of MiroDry™ Wound Matrix may gradually peel. Carefully remove any
remaining loose product around the edge as needed. Do not remove any remaining MiroDry™ Wound
Matrix that has integrated.
If the wound is free of infection and necrosis but not fully epithelialized, follow standard clinical protocol for additional application or therapy.
No posters match your selected filters. Remove some filtres, or reset them and start again.
Have a product to submit?
Be included in the most comprehensive wound care products directory
and online database.
Learn More